-
Extremity Chamber AOTI
-
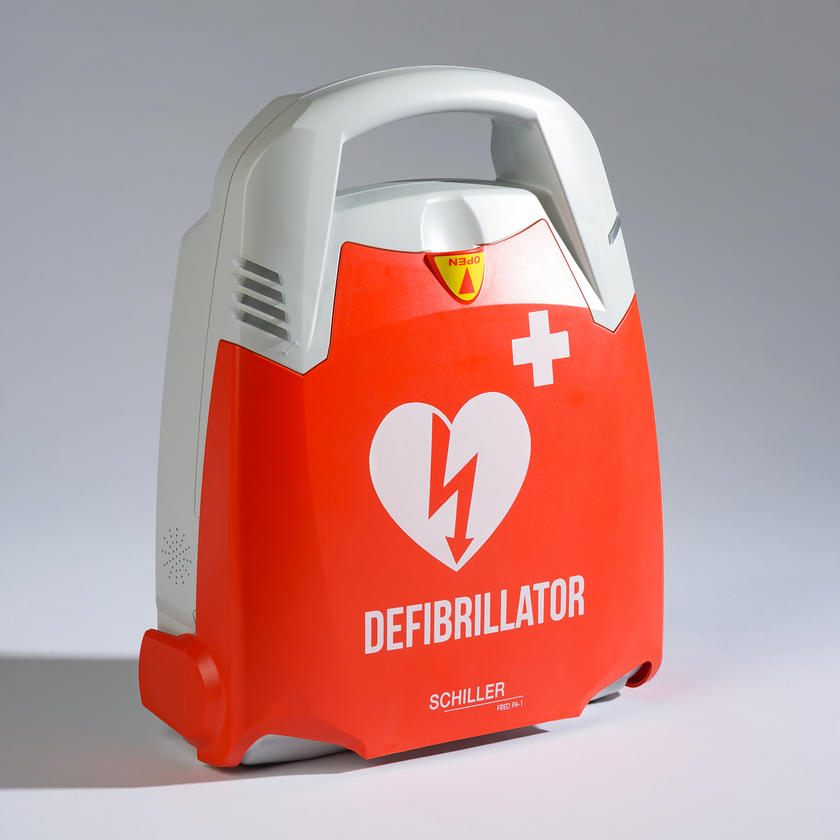
Fred PA-1
 By lifting the device cover, the FRED PA-1 starts up immediately and guides the rescuer step by step during the entire resuscitation process. The FRED PA-1 is available either as semi-automatic or fully automatic device.In its automatic version, the FRED PA-1 delivers the shock without any action of the rescuer. In its semi-automatic version, the FRED PA-1 prompts the rescuer to deliver the shock by pressing the shock button.
By lifting the device cover, the FRED PA-1 starts up immediately and guides the rescuer step by step during the entire resuscitation process. The FRED PA-1 is available either as semi-automatic or fully automatic device.In its automatic version, the FRED PA-1 delivers the shock without any action of the rescuer. In its semi-automatic version, the FRED PA-1 prompts the rescuer to deliver the shock by pressing the shock button. -
Holter Record ECG
 Atrial fibrillation/atrial flutter detection in zero seconds thanks to true P-wave analysis, easy, fast and hygienic to handle, flexible dual-battery concept for more than 14 days recording duration. This is the SCHILLER medilog®AR Holter.
Atrial fibrillation/atrial flutter detection in zero seconds thanks to true P-wave analysis, easy, fast and hygienic to handle, flexible dual-battery concept for more than 14 days recording duration. This is the SCHILLER medilog®AR Holter.The SCHILLER medilog®AR Holter is robust, shock- and splash-proof as well as easy to clean. Thanks to the dual-battery concept, the patients can be screened for more than 14 days without having
to see the doctor to change the batteries. Zero-second atrial fibrillation/atrial flutter detection based on true P-wave analysis is one of the engineering highlights of this Holter solution. -
Infusion Pump i5
 Syringe pump i5/i5-plus have been designed to facilitate a comprehensive solution for clinical application.
Syringe pump i5/i5-plus have been designed to facilitate a comprehensive solution for clinical application.
The stackable design greatly saves space and is easy to transfer.
3.5-inch touch screen and alarm light.
PBT plastic material for anti-corrosion.
On-line titration. (change infusion rate without pause)
Flow compensation to avoid errors caused by pipeline fatigue.
Anti-bolus functions to avoid adverse reactions caused by blood concentration.
KVO function.
5 levels alarm sound adjustable.
Multiple infusion modes.
Event log.
5 degrees bubble detection alarm. -
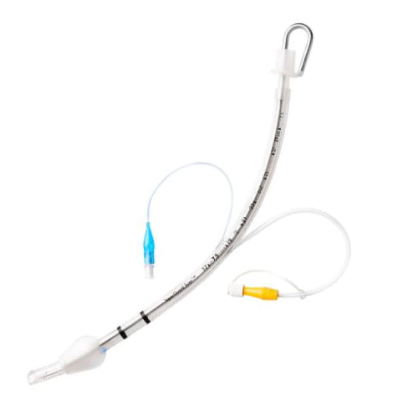
INTUBATION

Reduces ventilator-associated pneumonia (VAP) by an average of 50%1
The need to reduce ventilator associated events is the primary reason the Shiley™ evac oral endotracheal tube with TaperGuard™ cuff is critical for all patients on a ventilator in the ICU.
Advanced airway protection
Subglottic secretion drainage (SSD) helps remove oral and/or gastric secretions from above the endotracheal tube cuff before they can be aspirated. Such aspirations may lead to a very serious complication known as VAP or Ventilator Associated Pneumonia.
SSD must be done with a specialized endotracheal tube with a separate dorsal suction lumen located just above the cuff. Based upon clinical evidence, the following organizations recommend the use of SSD to reduce the incidence of VAP:
- SHEA Guidelines
- American Thoracic Society/ Infectious Diseases Society of America (ATS/IDSA) – Level I
- Centers for Disease Control (CDC) – Category II
- American Association of Critical Care Nurses (AACN)
- Agency for Healthcare Research and Quality (AHRQ)
-
McGRATH™ MAC Video Laryngoscope
-
Nebulizers and Accessories
 Our unique palladium vibrating mesh technology, Aerogen Vibronic® is a breakthrough in aerosol drug delivery and is at the heart of all our products
Our unique palladium vibrating mesh technology, Aerogen Vibronic® is a breakthrough in aerosol drug delivery and is at the heart of all our productsAs the world leader in acute care aerosol drug delivery, Aerogen have an extensive Intellectual Property Portfolio of more than 300 granted active and pending patents with coverage in multiple countries across the globe.
These patents are held in various Aerogen / Stamford Devices Ltd. Group companies. In addition Aerogen have co-licensed IP agreements with several institutions and MNC’s notably Novartis AG and Philips.
Aerogen uses IP within this portfolio to protect it’s market position, as it enhances the lives of millions of patients in more than 75 countries.
Certain Aerogen R&D initiatives are co-funded by the European Regional Development Fund and Enterprise Ireland as part of the Border, Midland and Western Regional Operational Programme 2014-2020. This R&D aims to develop novel nebulizer systems aimed at increasing R&D activity which will drive company development.
-
Nellcor™ Bedside SpO₂ Patient Monitoring System
 Monitor with confidence
Monitor with confidenceThe Nellcor™ bedside SpO2 patient monitoring system incorporates the latest Nellcor™ digital signal processing technology for accurate, reliable readings even during low perfusion and other forms of signal interference, providing clinicians with access to the most critical information regarding their patients’ respiratory status.
With improved functionality, including continuous SpO2 and pulse rate monitoring, trending data and SatSeconds alarm management, clinicians have the information they need to detect respiratory complications earlier and intervene sooner.
-
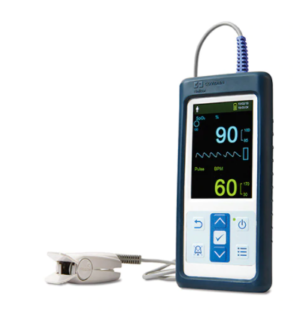
Nellcor™ Portable SpO₂ Patient Monitoring System, PM10N

Must-have features in a convenient, portable design
The Nellcor™ portable SpO2 patient monitoring system, PM10N, is a convenient, handheld monitor that is ideal for spot-checks or continuous monitoring in various healthcare and home use settings. Its ergonomic shape and simple design make it intuitive to use and simple to operate. Wherever you need accurate, reliable SpO2 measurements, count on the Nellcor™ portable SpO2 pulse oximeter to meet the challenge.
The monitoring system includes a vivid 3-inch color LCD screen, and connectivity to analytic tools and patient management systems. It is compatible with the entire line of Nellcor™ sensors with OxiMax™ technology and offers a robust monitoring feature set including SpO2, pulse rate, SatSeconds alarm management, pleth waveform, blip bar and tabular trend information.
The monitor incorporates Nellcor™ digital signal processing technology to deliver accurate, reliable SpO2 and pulse rate values even during low perfusion and signal interference, including patient motion.