-
Cardiovit AT-102 G2
 The CARDIOVIT AT-102 G2 features an 8” colour display with touch function keys. Moreover, its sealed alphanumeric keyboard ensures easy cleaning, making it highly suitable for the daily clinical routine. Smart Battery technology guarantees more than 8 hours of ECG recording.
The CARDIOVIT AT-102 G2 features an 8” colour display with touch function keys. Moreover, its sealed alphanumeric keyboard ensures easy cleaning, making it highly suitable for the daily clinical routine. Smart Battery technology guarantees more than 8 hours of ECG recording.Bidirectional communication allows for easy data access and fast transmission of ECG reports to EMR/HIS systems. Standard Wi-Fi and LAN connectivity, combined with ECG review on display and a large memory, supports paperless workflows and cost saving. When a paper report is required, customisable printouts can be performed on the internal high-resolution A4 thermal printer.
-
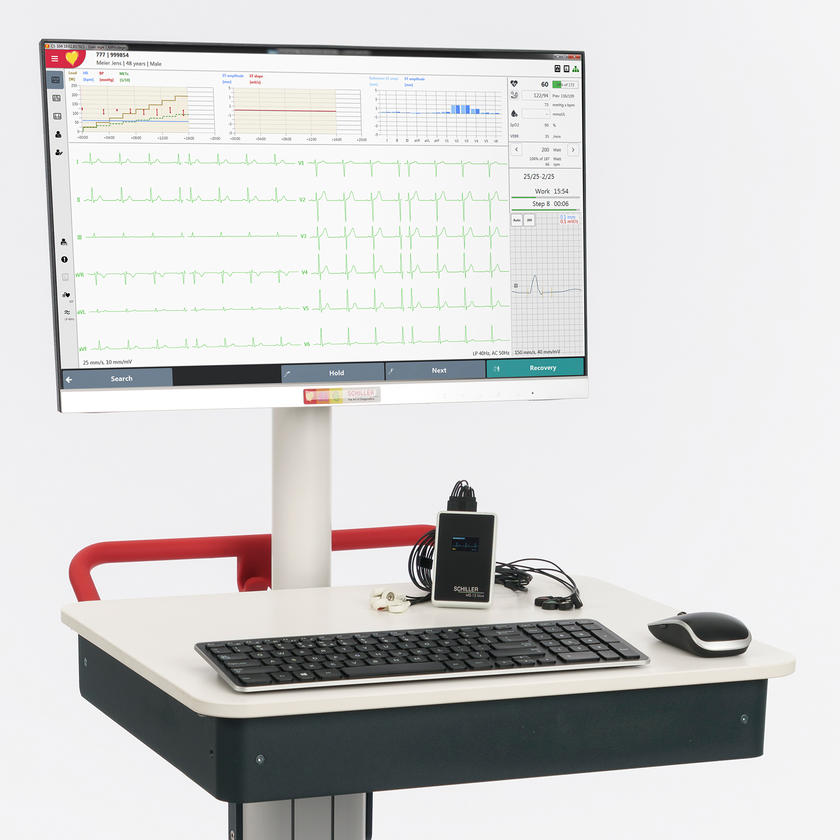
Cardiovit CS-104
 CARDIOVIT CS-104 combines all essential ECG features and desired possibilities in an integrated system.
CARDIOVIT CS-104 combines all essential ECG features and desired possibilities in an integrated system.With the CARDIOVIT CS-104, SCHILLER offers a complete ECG system for practices, clinics and hospitals. The unit includes a PC, a monitor, a trolley and a wireless (MS-12 blue) or wired (MS-12 USB) acquisition module. Everything is pre-installed and tested by SCHILLER, ready to be used in the clinical practice
-
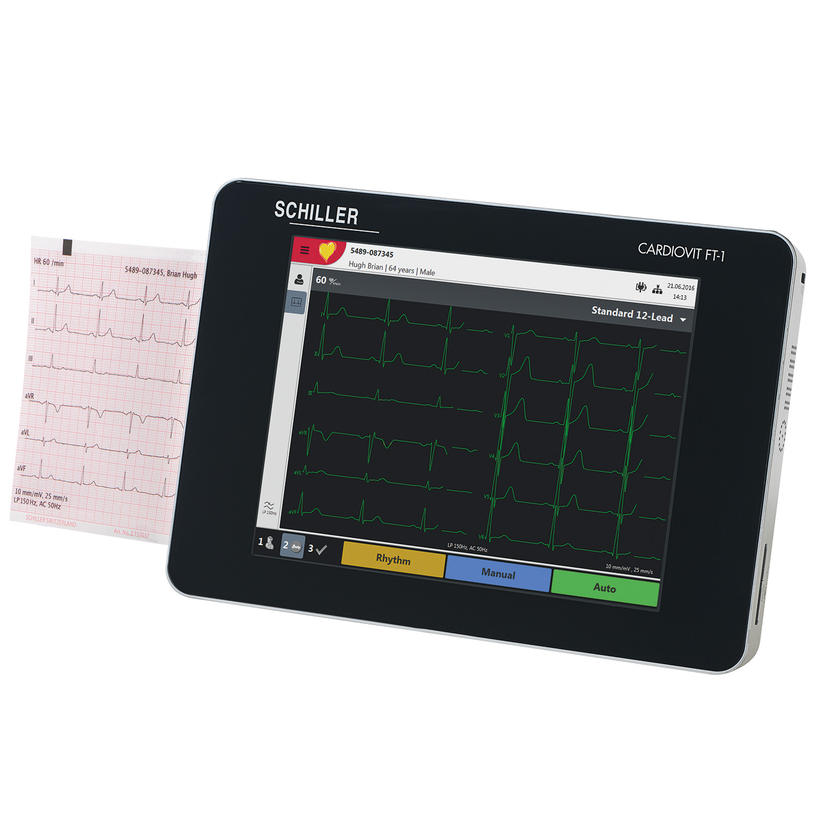
Cardiovit FT-1
 SCHILLER’s latest innovation is ultra portable, has an intuitive 8-inch high-resolution multi-touch screen, an integrated thermal printer and is FDA approved. Designed for users who value state-of-the-art technology, the CARDIOVIT FT-1 is a networked device providing connectivity and a paperless workflow. Please note: ETM with ETM Sport and CCAA are not cleared in USA.
SCHILLER’s latest innovation is ultra portable, has an intuitive 8-inch high-resolution multi-touch screen, an integrated thermal printer and is FDA approved. Designed for users who value state-of-the-art technology, the CARDIOVIT FT-1 is a networked device providing connectivity and a paperless workflow. Please note: ETM with ETM Sport and CCAA are not cleared in USA. -
Cardiovit MS-2015
 Advanced 12- or 16-channel ECG system
Advanced 12- or 16-channel ECG systemBased on the high-end touch screen techno-logy of the CARDIOVIT MS-2010, the CARDIOVIT MS-2015 offers an even larger 15-inch high-resolution colour display. It allows users to record, select and print highest quality 12- or 16-channel ECGs.
-
Chronic and Acute Dialysis solution
 Carpediem™ Cardio-Renal Pediatric Dialysis Emergency Machine
Carpediem™ Cardio-Renal Pediatric Dialysis Emergency MachineProvides continuous renal replacement therapy (CRRT) for acute kidney injury and fluid overloaded pediatric patients weighing 2.5 to 10 kgs.
-
CIRCULAR STAPLERS
 EEA™ Circular Stapler with Tri-Staple™ Technology
EEA™ Circular Stapler with Tri-Staple™ TechnologyCircular stapling now comes with Tri-Staple™ technology. That means greater leak protection1,†,‡ and greater perfusion allowed into the staple line.2,§,Ω
-
Cool-tip™ RF Ablation System E Series
 OVERVIEW
OVERVIEWSimple and intuitive design with added safety features
For thermal ablation of soft-tissue tumors, we offer the Cool-tip™ RF ablation system E Series*.
The E Series improves on the trusted platform of the Cool-tip™ RFA system, preferred worldwide for its straight-needle design and maximum energy delivery, making it possible to create larger ablation zones in less time and with less charring than other RFA systems.
*The Cool-tip™ RF ablation system E Series is not available for sale in the United States. All Cool-tip™ products bear the CE Mark.
-
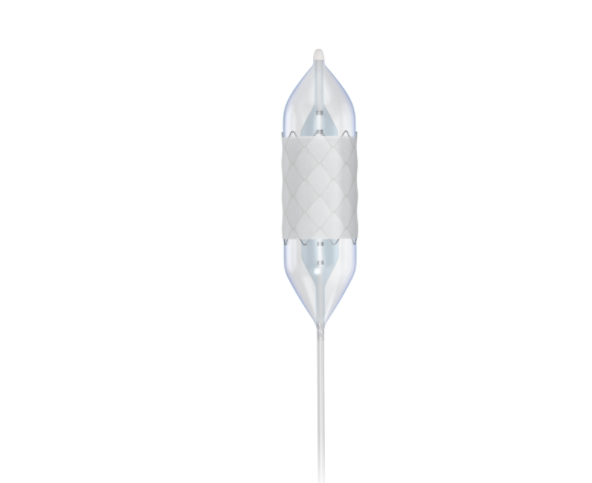
Covered CP Stent™
 The Covered CP Stent™ is manufactured by hand using high-quality materials. This device has undergone rigorous testing to achieve its dual indication labeling. The Covered CP Stent™ has a proven safety record through data recorded from both clinical studies as well as post-market feedback. The stent is available in both unmounted and pre-mounted (on a BIB® catheter) configurations. The 8 zig Covered CP Stent™ has an expansion range of 12.0 – 24.0 mm. The 10 zig Covered CP Stent™ has an expansion range of 26.0 – 30.0 mm. Thanks to its considerable capacity for expansion, the Covered CP Stent™ can be re-dilated to accommodate a patient’s natural growth, potentially reducing the need for additional stent implantation.
The Covered CP Stent™ is manufactured by hand using high-quality materials. This device has undergone rigorous testing to achieve its dual indication labeling. The Covered CP Stent™ has a proven safety record through data recorded from both clinical studies as well as post-market feedback. The stent is available in both unmounted and pre-mounted (on a BIB® catheter) configurations. The 8 zig Covered CP Stent™ has an expansion range of 12.0 – 24.0 mm. The 10 zig Covered CP Stent™ has an expansion range of 26.0 – 30.0 mm. Thanks to its considerable capacity for expansion, the Covered CP Stent™ can be re-dilated to accommodate a patient’s natural growth, potentially reducing the need for additional stent implantation. -
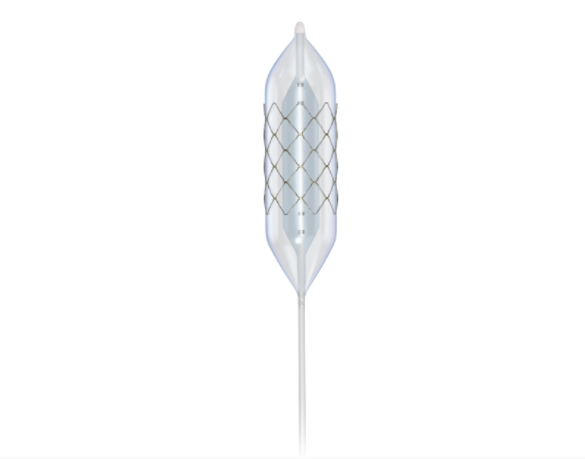
CP Stent®
 The CP Stent® is manufactured by hand using high-quality materials. The CP Stent® has a proven safety record through data recorded from both clinical studies as well as post-market feedback. The stent is available in both unmounted and pre-mounted (on a BIB® catheter) configurations. The 8 zig CP Stent® has an expansion range of 12.0 – 24.0 mm. The 10 zig CP Stent® has an expansion range of 26.0 – 30.0 mm. Thanks to its considerable capacity for expansion, the CP Stent® can be re-dilated to accommodate a patient’s natural growth, potentially reducing the need for additional stent implantation.
The CP Stent® is manufactured by hand using high-quality materials. The CP Stent® has a proven safety record through data recorded from both clinical studies as well as post-market feedback. The stent is available in both unmounted and pre-mounted (on a BIB® catheter) configurations. The 8 zig CP Stent® has an expansion range of 12.0 – 24.0 mm. The 10 zig CP Stent® has an expansion range of 26.0 – 30.0 mm. Thanks to its considerable capacity for expansion, the CP Stent® can be re-dilated to accommodate a patient’s natural growth, potentially reducing the need for additional stent implantation.