-
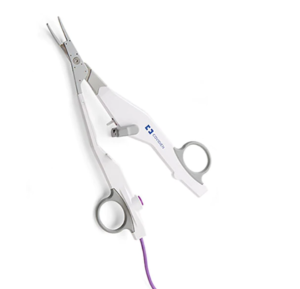
LigaSure™ Exact Dissector

OVERVIEW
Introducing the LigaSure™ exact dissector
Built on reliable LigaSure™ technology, our newest surgical advancement offers faster cooling times2 with superior access and more precise dissection.1,†
-
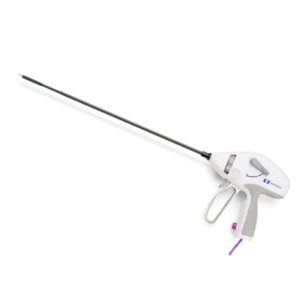
McGRATH™ MAC Video Laryngoscope
-
Mechanical Beds
General Technical Specifications :
- Epoxy powder coated steel Two sections or four sections.
- Stainless steel (one) IV pole & (two) IV pole mounts .
- 4 Bumpers for protection.
- Washable 12 cm water proof covered, high density, soft foam mattress
- Patient area: (L x W x H ) 200 x 90 x 50 cm . without mattress .
- Overall dimension: (L x W x H ) 220 x 105 x 50 cm .
- 5 inch x 4 swivel castors (two of them with brakes).
- Easily removable (One step), ABS head and foot board.
- Up to 200 KG.
- Metal sheet and Foldable side rails .
Type of Models :
- Half Fowler Bed.
- Full Fowler Bed.
Download PDF
-
MESH PRODUCTS

Innovation that matters
Innovation meets performance in this comprehensive line of synthetic mesh for hernia surgery. Accommodating broad flexibility in preferred techniques and in inguinal, ventral, umbilical, open and laparoscopic surgical procedures, our mesh portfolio combines superior clinical efficiency with positive patient outcomes. This depth and diversity of design allows surgeons to take a patient-specific approach to hernia repair while enjoying the enhanced value of line standardization.
-
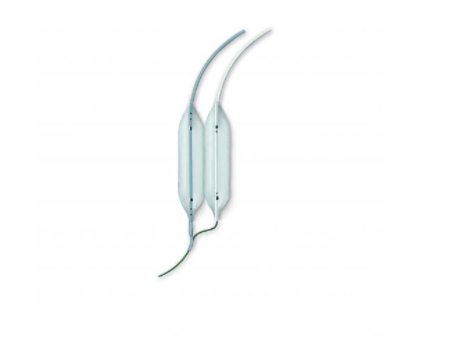
Mitral Dilatation Kit
 The Bonhoeffer Multi-Track Mitral Dilatation Kit was developed in collaboration with Dr. Philipp Bonhoeffer and is an innovative and well-established system for Mitral Dilatation. This consists of a double balloon technique while using a simple, single guidewire approach. The innovative approach was invented to solve the problem of the mismatch between the round shape of a single balloon and the oval mitral orifice. The technique achieves consistently higher valve areas post dilatation.
The Bonhoeffer Multi-Track Mitral Dilatation Kit was developed in collaboration with Dr. Philipp Bonhoeffer and is an innovative and well-established system for Mitral Dilatation. This consists of a double balloon technique while using a simple, single guidewire approach. The innovative approach was invented to solve the problem of the mismatch between the round shape of a single balloon and the oval mitral orifice. The technique achieves consistently higher valve areas post dilatation. -
Nebulizers and Accessories
 Our unique palladium vibrating mesh technology, Aerogen Vibronic® is a breakthrough in aerosol drug delivery and is at the heart of all our products
Our unique palladium vibrating mesh technology, Aerogen Vibronic® is a breakthrough in aerosol drug delivery and is at the heart of all our productsAs the world leader in acute care aerosol drug delivery, Aerogen have an extensive Intellectual Property Portfolio of more than 300 granted active and pending patents with coverage in multiple countries across the globe.
These patents are held in various Aerogen / Stamford Devices Ltd. Group companies. In addition Aerogen have co-licensed IP agreements with several institutions and MNC’s notably Novartis AG and Philips.
Aerogen uses IP within this portfolio to protect it’s market position, as it enhances the lives of millions of patients in more than 75 countries.
Certain Aerogen R&D initiatives are co-funded by the European Regional Development Fund and Enterprise Ireland as part of the Border, Midland and Western Regional Operational Programme 2014-2020. This R&D aims to develop novel nebulizer systems aimed at increasing R&D activity which will drive company development.