-
Holter Record ECG
 Atrial fibrillation/atrial flutter detection in zero seconds thanks to true P-wave analysis, easy, fast and hygienic to handle, flexible dual-battery concept for more than 14 days recording duration. This is the SCHILLER medilog®AR Holter.
Atrial fibrillation/atrial flutter detection in zero seconds thanks to true P-wave analysis, easy, fast and hygienic to handle, flexible dual-battery concept for more than 14 days recording duration. This is the SCHILLER medilog®AR Holter.The SCHILLER medilog®AR Holter is robust, shock- and splash-proof as well as easy to clean. Thanks to the dual-battery concept, the patients can be screened for more than 14 days without having
to see the doctor to change the batteries. Zero-second atrial fibrillation/atrial flutter detection based on true P-wave analysis is one of the engineering highlights of this Holter solution. -
INFINITI™ Diagnostic Catheter

Ideal for coronary angiography. The INFINITI™ Diagnostic Catheter incorporates the proprietary VESTAN™ Nylon to deliver exceptional responsiveness and flow rates, optimal torque, and shape retention.
Diagnose
- EMERALD™ Diagnostic Guidewire
- AQUATRACK™ Hydrophilic Nitinol Guidewire
- INFINITI™ Diagnostic Catheter
- SUPERTORQUE™ Catheters
- 7F HIGHFLOW™ Catheter
- ANGIOGUARD™ RX Guidewire System
-
Infusion Pump i5
 Syringe pump i5/i5-plus have been designed to facilitate a comprehensive solution for clinical application.
Syringe pump i5/i5-plus have been designed to facilitate a comprehensive solution for clinical application.
The stackable design greatly saves space and is easy to transfer.
3.5-inch touch screen and alarm light.
PBT plastic material for anti-corrosion.
On-line titration. (change infusion rate without pause)
Flow compensation to avoid errors caused by pipeline fatigue.
Anti-bolus functions to avoid adverse reactions caused by blood concentration.
KVO function.
5 levels alarm sound adjustable.
Multiple infusion modes.
Event log.
5 degrees bubble detection alarm. -
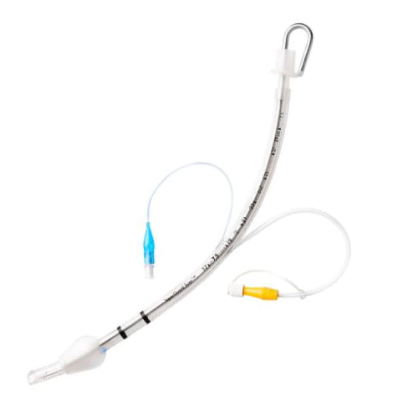
INTUBATION

Reduces ventilator-associated pneumonia (VAP) by an average of 50%1
The need to reduce ventilator associated events is the primary reason the Shiley™ evac oral endotracheal tube with TaperGuard™ cuff is critical for all patients on a ventilator in the ICU.
Advanced airway protection
Subglottic secretion drainage (SSD) helps remove oral and/or gastric secretions from above the endotracheal tube cuff before they can be aspirated. Such aspirations may lead to a very serious complication known as VAP or Ventilator Associated Pneumonia.
SSD must be done with a specialized endotracheal tube with a separate dorsal suction lumen located just above the cuff. Based upon clinical evidence, the following organizations recommend the use of SSD to reduce the incidence of VAP:
- SHEA Guidelines
- American Thoracic Society/ Infectious Diseases Society of America (ATS/IDSA) – Level I
- Centers for Disease Control (CDC) – Category II
- American Association of Critical Care Nurses (AACN)
- Agency for Healthcare Research and Quality (AHRQ)